
A friendship between scientists is a finely tuned machine. It’s powered by collaboration, intellectual banter, and, sometimes, novel discoveries that have the potential to dramatically improve lives. That’s no small talk.
For Ryan Bavis of Bates and Peter MacFarlane of Case Western Reserve University, 20 years of friendship — sabbatical projects, co-written papers, and conferences spent rooming together — has culminated with the discovery of a novel animal model that might provide groundbreaking insights into the cause of sudden infant death syndrome.
Last year, the National Institutes of Health awarded the scientists a $2.8 million grant to study the model over five years. MacFarlane is the project’s principal investigator, and $307,495 is allocated to Bates. Dr. Richard Martin of Rainbow Babies and Children’s Hospital and professor at CWRU’s School of Medicine is also a co-investigator on the project.
With their research, MacFarlane, an associate professor in the Department of Pediatrics at CWRU and the Division of Neonatology at the University Hospitals Rainbow Babies and Children’s Hospital, and Bavis, the Papaioanou Professor of Biological Sciences at Bates, are tackling a subject that is notoriously difficult to study.

Sudden infant death syndrome, or SIDS, refers to the sudden and unexplainable death of a seemingly healthy infant and is the number one cause of death for infants between one month and one year of age. In the U.S., about 1,500 infants die of SIDS each year. SIDS is a diagnosis of exclusion; it’s only identified as a cause of death once all other possible causes have been ruled out.
Though its frequency has declined in recent decades, SIDS remains a source of anxiety and heartbreak for parents. Scientists have ideas about what causes SIDS but no method to preventatively treat it or identify which infants may be vulnerable.
This is not for lack of trying; scientists struggle to find participants to study a syndrome that is so rare — 38 SIDS deaths per 100,000 live births were documented in the U.S. in 2020 — and traumatizing for families.
“It’s just such a sensitive thing,” Bavis says. “I can’t imagine anything more traumatic than the unexpected death of a seemingly healthy child during that first year of life.”
Especially when researching rare afflictions, scientists often turn to animal models that mimic human pathophysiology, the bodily changes that occur as a result of disease or injury. Until now, scientists had not identified a reliable animal model for SIDS. Bavis and MacFarlane are aiming to change that.
The two met and became friends through scientific conferences and their shared research interests in breathing and respiratory and nervous systems. They have collaborated on various scientific projects over the years and are now investigating a newly-discovered animal model that may offer insight into the causes, prevention, and hallmark features of SIDS.
“We think that we have an animal model that recapitulates a lot of what’s going on in sudden infant death syndrome, which gives us a chance to test some of the hypotheses that have been around for a long time but also gives us the opportunity to generate new hypotheses,” Bavis says.

Like many scientific advances, the animal model was discovered accidentally. While conducting a different experiment where he exposed rats to low levels of oxygen, as part of his research on clinical scenarios commonly experienced by preterm infants, MacFarlane discovered that several — but not all — of the animals died spontaneously several days after exposure.
Thinking it was a fluke result, MacFarlane repeated the experiment and soon discovered abnormalities in the deceased animals consistent with those seen in infants who have died from SIDS.
“Sometimes you follow the unexpected results as well, and you see what they can evolve into,” MacFarlane says.
How SIDS happens
MacFarlane’s experiments with the rats seemed to mimic the Triple-Risk Model, the most widely accepted hypothesis to explain how SIDS occurs in humans. Proposed by researchers Hannah Kinney and James J. Filiano, this model posits that three factors must be present for SIDS to occur.
First, the affected infant must have an underlying vulnerability. Second, the infant must be in a critical stage of development (most SIDS victims are 2 to 4 months old). Third, the infant must experience some kind of outside stressor made fatal by the initial underlying vulnerability.
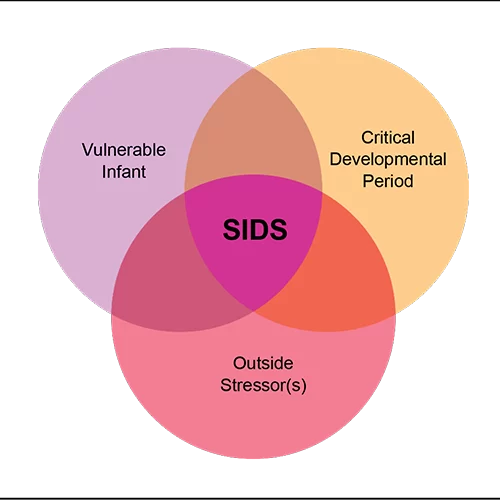
The well-accepted concept for an underlying cause of death in SIDS involves abnormalities in the brainstem, which contains neurons important for controlling and regulating breathing. Unfortunately, nobody knows what causes the abnormalities.
Bavis and MacFarlane propose that the brainstem abnormalities arise from abnormal communication from the child’s carotid body, a peripheral nervous system organ in the neck which monitors carbon dioxide and oxygen in the blood. The carotid body connects peripheral parts of the body to the brainstem and provides important inputs to regulate breathing, among other autonomic functions.
In infants who have died of SIDS, scientists have observed consistent brainstem abnormalities within microglia, the primary immune defense cells in the central nervous system, and serotonin-expressing neurons, which regulate breathing and other bodily functions.
Scientists are not certain how these abnormalities are created. Bavis and MacFarlane hypothesize that they could be the result of an infant’s early experience of hypoxia, in which oxygen levels in the body’s tissues and organs are too low.
“There may be some kind of a genetic component, but we think that environmental situations during our early development can also lead to the vulnerability,” Bavis says.
These abnormalities inhibit an infant’s ability to regulate breathing, making them vulnerable to outside stressors.
For example, when a sleeping infant rolls from their back to their stomach, facing their mattress and therefore breathing in the carbon dioxide that they are exhaling instead of fresh air, a healthy carotid body and brainstem system would alert the infant to reposition themselves to breathe new air.
An infant with a vulnerable carotid body and brainstem system, however, may not receive the alert to reposition themselves. Breathing in the same air that they are exhaling instead of fresh oxygen, the child would risk fatal injury characteristic of SIDS.
Babies who are born prematurely, sleep on their stomachs, sleep with stuffed animals and bedding, or are exposed to maternal alcohol and cigarette use are all more at risk of dying from SIDS.
Animal models in action
In their project, Bavis and MacFarlane propose an animal model using rats that mimics how SIDS, via the conditions of the Triple-Risk Model, occurs in infants. The model also presents the same brainstem and carotid body abnormalities seen in many human cases. If deemed to be effective, it could be an invaluable resource for scientists and doctors working to better understand SIDS.
“Having all these combined numbers of physiological features that mimic the human situation all within one model is a really unique opportunity that allows us to really put all of the pieces of the puzzle together,” MacFarlane says.
Additionally, MacFarlane and Bavis will use the animal model to look for a possible third brainstem abnormality within the extracellular matrix, a network of minerals that supports cells.
“To our knowledge, [extracellular matrix abnormalities have] never been looked for in human SIDS cases,” Bavis says. “One of the exciting things about our model is it can actually generate new hypotheses.”
The two also propose that abnormalities in the brainstem are the result of the carotid body’s signals to the brainstem being interrupted. This would cause the brain to receive inaccurate information about oxygen and carbon dioxide levels, and the child would be unable to properly respond to their surrounding oxygen conditions.
To test their hypotheses, the scientists will expose rats to low oxygen levels, causing them to enter hypoxic states, and observe if the effects mimic the abnormalities consistent with SIDS pathology. Bavis will measure how the animals’ post-experiment carotid bodies respond to oxygen through electrophysiological tests that assess the electrical properties of cells and tissues, while MacFarlane is focused on the effects in the central nervous system and the brainstem abnormalities.

Comprehension as prevention
In addition to verifying the animal model’s efficacy, the scientists hope to identify preventative treatments for SIDS.
For one, MacFarlane will be testing a drug that could prevent the hallmark microglial brainstem abnormalities and therefore decrease the infant’s vulnerability to outside stressors. If the drug is successful in rats, scientists could research its efficacy in humans.
“If we can target that upstream microglia, we can prevent some of the inflammatory response and hopefully those infants go on to have a normal, developing respiratory system that keeps them safe from SIDS,” MacFarlane said.
MacFarlane will also be looking for biomarkers in rats’ blood, urine, and other fluids that could indicate which animals are more vulnerable to developing abnormalities or dying from SIDS-like injury. If successfully identified in animals and subsequently humans, biomarkers could be used to create SIDS screening tests for infants.
“Understanding why someone died, there’s useful information there, but if you can’t do anything to prevent it, then it’s just not as exciting and fulfilling,” Bavis says. “I think those biomarkers are the most exciting part of this project.”
Looking forward
MacFarlane and Bavis’ research has big goals — confirm the efficacy of the animal model, identify new abnormalities, and work toward preventative SIDS treatments — and big potential. SIDS experts recognize the impact the project could have; Dr. Hannah Kinney, co-creator of the Triple-Risk Model, wrote a letter of support for the grant proposal and meets virtually with the research team each month.
“The fact that there was the buy-in from the actual SIDS research community — that our animal model was something that was worth investing in — was really exciting for us,” Bavis says.
If it successfully supports its hypotheses, the study could lay the groundwork for new research with human subjects, a much deeper understanding of what causes SIDS, and the chance to save families from the grief of losing a child. From a syndrome shrouded in mystery, scientists are chipping away at illuminating clues.
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD111415. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.




